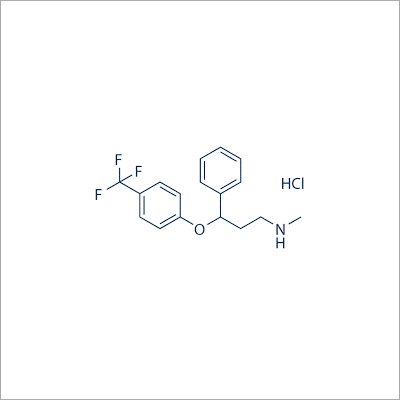
Fluoxetine HCL IP
Fluoxetine HCL IP Specification
- Loss on Drying
- 0.5%
- Heavy Metal (%)
- 0.001%
- Molecular Formula
- C17H18F3NO HCl
- Storage
- Store in a cool, dry place, protected from light
- Place of Origin
- India
- Molecular Weight
- 345.79 g/mol
- Boiling point
- Not Applicable (decomposes before boiling)
- HS Code
- 29224990
- Melting Point
- 165-166 C
- Residue on Ignition
- 0.1%
- EINECS No
- 259-031-2
- Assay
- >=99% (on dried basis)
- Ph Level
- 4.5 to 6.0 (1% solution)
- Moisture (%)
- 0.5%
- Particle Size
- Not less than 90% passes through 60 mesh
- Other Names
- N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1-amine hydrochloride
- CAS No
- 56296-78-7
- Type
- Active Pharmaceutical Ingredient
- Grade
- Pharmaceutical Grade
- Usage
- Antidepressant, Treatment of major depressive disorder, Obsessive-compulsive disorder
- Purity
- >=99%
- Appearance
- White to off-white crystalline powder
- Application
- Pharmaceutical Intermediate, API
- Raw Material
- Synthesized Chemical
- Smell
- Odorless
- Color
- White
- Form
- Powder
- Identification
- Complies with IP/USP standard
- Shelf Life
- 36 months
- Sterility
- Non-sterile
- Optical Rotation
- Not more than 1
- Solubility
- Freely soluble in water and alcohol
- Endotoxin Level
- NA (Not applicable for API raw material use)
- Packaging
- HDPE Drum / Double LDPE liner, 25 kg net
- Residual Solvents
- Complies with ICH guidelines
- Related Substances
- Maximum single impurity 0.1%
- Microbial Limits
- Total aerobic microbial count <1000 CFU/g, Yeast & moulds <100 CFU/g
- TLC/Chromatography Purity
- >99% by HPLC/TLC
Fluoxetine HCL IP Trade Information
- Minimum Order Quantity
- 5 Kilograms
- Payment Terms
- Letter of Credit (L/C), Cash Against Delivery (CAD), Cash on Delivery (COD), Cash in Advance (CID), Cheque, Cash Advance (CA)
- Supply Ability
- 10000 Kilograms Per Month
- Delivery Time
- 15 Days
- Sample Available
- Yes
- Main Domestic Market
- All India
- Certifications
- GMP
About Fluoxetine HCL IP
These chemicals are antidepressant agents belonging to the selective serotonin reuptake inhibitors. We have uniquely positioned ourselves as a dependable firm, engaged in offering our clients Fluoxetine Chemicals. Our expert workforce uses superior grade compounds to process these chemicals. They are used to treat major depressive disorder (MDD), moderate to severe bulimia nervosa and obsessive-compulsive disorder (OCD). These chemicals are also effective in panic disorder with or without agoraphobia. Fluoxetine Chemicals are used in combination with olanzapine for treatment-resistant or bipolar I depression. These chemicals are packed in moisture proof containers and are available in different quantity packaging options.
FLUOXETINE:- Fluoxetine Hydrochloride is Benzenepropanmine, N-methyl- -[4-(trifluoromethyl) phenoxy]- ,hydrochloride
Formula: C17H18F3NO,HCl
Category: Antidepressant.
Mol. Wt.: 345.79
| Analysis | Specification |
| Description | White to off-white Crystalline Powder |
| Solubility
| Sparingly soluble in water and in dichloromethane, freely soluble in alcohol and in methanol. Practically insoluble in ether. |
| Identification:
A: B: | Byinfra-red absorption spectrum
Gives the reactions ofchlorides, |
| Water | Not More than 0.5 % |
| Heavy Metals | Not more than 0.003 % |
| Sulphated Ash | Not more than 0.1% |
| Related Compound | Total impurities NMT 0.5 % |
| Organic Volatile Impurity | To Comply the test |
| Assay | Fluoxetine Hydrochloride contains not less than 98.0 per cent and not more than 102.0 per cent of:C17H18F3NO,HCl, calculated ontheanhydrous basis. |
Superior Quality and Purity Assurance
Fluoxetine HCL IP complies with stringent quality benchmarks, including over 99% purity confirmed by HPLC/TLC chromatography. Meticulous controls on impurities, residual solvents, moisture content, and heavy metals ensure pharmaceutical-grade consistency and safety, making it suitable for critical medical applications.
Versatility in Formulation and Application
The products excellent solubility in water and alcohol, along with a finely controlled particle size (over 90% passing through 60 mesh), supports seamless integration into various dosage forms such as tablets and capsules. Its robust chemical stability enhances product shelf life up to 36 months when stored appropriately.
Robust Packaging and Global Standards
To preserve efficacy and integrity during transit and storage, Fluoxetine HCL IP comes securely packed in HDPE drums with double LDPE liners. Every batch is identified to comply with IP/USP pharma-grade requirements and meets international export standards, making it ideal for global pharmaceutical manufacturing.
FAQs of Fluoxetine HCL IP:
Q: How should Fluoxetine HCL IP be stored for optimal stability?
A: Fluoxetine HCL IP should be stored in a cool, dry place, protected from light, to maintain its 36-month shelf life and prevent degradation of its chemical properties.Q: What pharmaceutical applications is Fluoxetine HCL IP suitable for?
A: This high-purity API is used primarily in the manufacture of antidepressant medications for major depressive disorder and obsessive-compulsive disorder, thanks to its regulatory compliance and excellent solubility.Q: When does the product shelf life commence and what is its duration?
A: The 36-month shelf life begins from the date of manufacture, provided the product is kept in recommended storage conditions and remains in its original, tightly sealed packaging.Q: Where is Fluoxetine HCL IP manufactured and what quality standards does it meet?
A: Fluoxetine HCL IP is manufactured in India and complies with IP and USP standards, ensuring it meets stringent pharmaceutical-grade quality requirements for global markets.Q: What is the typical process for ensuring purity and safety in Fluoxetine HCL IP production?
A: Purity and safety are guaranteed through advanced synthesis techniques, extensive impurity profiling, consistent HPLC/TLC analysis, and adherence to ICH guidelines for residual solvents and stringent microbial limits.Q: How can Fluoxetine HCL IP benefit pharmaceutical manufacturers?
A: With its high assay value (99%), low impurity content, robust packaging, and excellent solubility, it offers reliable consistency and maximizes formulation flexibility for pharmaceutical companies.Q: What regulations and standards does Fluoxetine HCL IP adhere to for export?
A: It adheres to international standards, including IP/USP identification, ICH guidelines for residual solvents, and meets the HS Code 29224990, making it suitable for export and use worldwide as a pharmaceutical raw material.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Fluoxetine HCL Category
Fluoxetine Hydrochloride USP
Price 4500 INR / Kilograms
Minimum Order Quantity : 5 Kilograms
Molecular Weight : 345.8 g/mol
Moisture (%) : 0.5%
Usage : Antidepressant, treatment of major depressive disorder, obsessive compulsive disorder, and panic disorder
Application : Other, Active Pharmaceutical Ingredient (API)
GST : 24AABCP1568Q1Z0
|
 |
PALAM PHARMA PVT. LTD.
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |



 Send Inquiry
Send Inquiry