Dicyclomine Hydrochloride USP
Dicyclomine Hydrochloride USP Specification
- Loss on Drying
- 0.5%
- Particle Size
- Complies with specification
- Molecular Weight
- 345.95 g/mol
- HS Code
- 29333990
- Storage
- Store in a cool, dry, well ventilated area away from heat and moisture
- Taste
- Bitter
- Smell
- Odorless
- Molecular Formula
- C19H35NO2HCl
- Heavy Metal (%)
- 0.002%
- Shelf Life
- 5 years when properly stored
- Color
- White
- Ph Level
- 4.0 to 6.0 (in 5% aqueous solution)
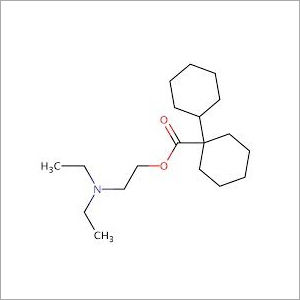
- Structural Formula
- Available on request
- Solubility
- Freely soluble in water; soluble in alcohol
- Medicine Name
- Dicyclomine Hydrochloride USP
- Type
- Active Pharmaceutical Ingredient
- Grade
- USP
- Purity(%)
- 99%
- Appearance
- White to almost white crystalline powder
- Physical Form
- Solid
Dicyclomine Hydrochloride USP Trade Information
- Minimum Order Quantity
- 5 Kilograms
- Supply Ability
- 10000 Kilograms Per Month
- Delivery Time
- 15 Days
- Main Domestic Market
- All India
About Dicyclomine Hydrochloride USP
These chemicals are used to treat a certain type of intestinal problem called irritable bowel syndrome. Creating a striking mark in the industry, we have earned the name of being a highly reliable organization engaged in offering Dicyclomine Hydrochloride USP Chemicals. They are a muscarinic antagonist used as an antispasmodic and in urinary incontinence. These chemicals have little effect on glandular secretion or the cardiovascular system. They are known for having some local anesthetic properties. To comply with set safety norms, we use fionest quality compounds to process these chemicals. Dicyclomine Hydrochloride USP Chemicals are effective in gastrointestinal, biliary, and urinary tract spasms.
Dicyclomine Hydrochloride IP/BP/USP Dicyclomine Hydrochloride is 2-diethylaminoethyl- bicyclohexyl-1-carboxylate hydrochloride
Formula: C19H35NO2, HCl
Category: Antispasmodic.
Mol. Wt.: 345.95
Dose: 30 to 60 mg daily, in divided doses. If used as an oral solution and the solution is required to be diluted, the diluted solution should be freshly prepared.
| Analysis | Specification |
| Description | White or almost white, crystalline powder; odourless or almost odourless. |
| Solubility | Freely soluble in ethanol (95%) and in chloroform; soluble in water; practically insoluble in ether. |
| Identification: | |
| A: | By infra-red absorption spectrum |
| B: | To 3 ml of a 0.1% w/v solution of sodium dodecyl sulphate, add 5 ml of chloroform and 0.05 ml of a 0.25% w/v solution of methylene blue, mix gently and allow to separate; the chloroform layer is blue. Add 20 mg of the substance being examined dissolved in 2 ml of water, mix gently and allow to separate; the aqueous layer is blue and the chloroform layer is colourless. |
| C: Chloride Test | Dissolve 10 mg in 5 ml of water and add 0.2 ml of 2M nitric acid and 0.5 ml of silver nitrate solution; a white precipitate is produced. |
| D: Melts | Between 172o and 174o. |
| Related Substances | Any secondary spot in the chromatogram obtained with solution (1) is not more intense than the spot in the chromatogram obtained with solution (2). |
| Sulphated Ash | Not more than 0.1%. |
| Loss on Drying | Not more than 1.0%, determined on 1 g by drying in an oven at 105o |
| Assay | Dicyclomine Hydrochloride contains not less than 99.0 per cent and not more than 101.0 per cent of C19H35NO2,HCl, calculated with reference to the dried substance. |
Exceptional Purity and Compliance
Dicyclomine Hydrochloride USP boasts a purity level of at least 99%, making it highly suitable for pharmaceutical applications requiring stringent quality standards. Its USP grade certification assures users of its reliability and conformity with international pharmacopeial standards. Each batch is produced to meet rigorous specifications for physical properties and chemical composition.
Reliable Physical and Chemical Attributes
This active pharmaceutical ingredient appears as a white to almost white crystalline powder and is odorless with a distinctly bitter taste. It has a molecular weight of 345.95 g/mol and is freely soluble in water, ensuring ease of processing in various drug formulations. Its pH range of 4.0 to 6.0 supports diverse applications in pharmaceutical manufacturing.
Storage and Longevity
To maintain its stability and effectiveness, Dicyclomine Hydrochloride USP should be stored in a cool, dry, and well-ventilated place, away from sources of heat and moisture. When stored as recommended, it remains stable and effective for up to 5 years, offering manufacturers and suppliers prolonged usability without compromising quality.
FAQs of Dicyclomine Hydrochloride USP:
Q: How should Dicyclomine Hydrochloride USP be stored to maintain its quality?
A: Dicyclomine Hydrochloride USP should be stored in a cool, dry, and well-ventilated area, away from heat and moisture. This ensures the product remains stable and retains its quality throughout its 5-year shelf life.Q: What is the main usage of Dicyclomine Hydrochloride USP as an active pharmaceutical ingredient?
A: Dicyclomine Hydrochloride USP is primarily used in the pharmaceutical industry for the formulation of medications. Its high purity and compliance with USP standards make it a preferred ingredient in the production of various pharmaceutical drugs.Q: When does Dicyclomine Hydrochloride USP expire and how is its shelf life determined?
A: The shelf life of Dicyclomine Hydrochloride USP is up to 5 years when stored as per the recommended conditions. The expiration is determined based on stability studies to ensure the product maintains its purity and efficacy over time.Q: Where is Dicyclomine Hydrochloride USP manufactured and exported from?
A: Dicyclomine Hydrochloride USP is manufactured, supplied, and exported from India, with reliable quality management to meet international standards.Q: What is the benefit of using Dicyclomine Hydrochloride USP with high purity in pharmaceutical manufacturing?
A: High-purity Dicyclomine Hydrochloride USP guarantees the safety, efficacy, and consistency of end pharmaceutical products, reducing the risk of impurities and ensuring regulatory compliance.Q: How can Dicyclomine Hydrochloride USP be identified physically and chemically?
A: Dicyclomine Hydrochloride USP is a white to almost white, odorless crystalline powder with a bitter taste, freely soluble in water and alcohol. It complies with specific particle size and loss on drying specifications, ensuring consistent quality.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Dicyclomine HCL Category
Dicyclomine Hydrochloride BP
Minimum Order Quantity : 5 Kilograms
Loss on Drying : Less than 0.5%
Usage : Used as anticholinergic, antispasmodic
HS Code : 29221990
Molecular Weight : 345.95 g/mol
Shelf Life : 5 years
Dicyclomine Hydrochloride IP
Minimum Order Quantity : 5 Kilograms
Loss on Drying : Not more than 0.5%
Usage : Antispasmodic agent in the treatment of irritable bowel syndrome and related conditions
HS Code : 29224990
Molecular Weight : 345.95 g/mol
Shelf Life : 5 years
GST : 24AABCP1568Q1Z0
|
 |
PALAM PHARMA PVT. LTD.
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |


 Send Inquiry
Send Inquiry