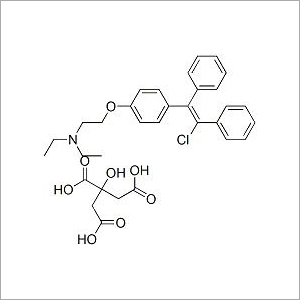
Clomiphene Citrate USP
Clomiphene Citrate USP Specification
- HS Code
- 29333990
- Residue on Ignition
- Not more than 0.1%
- Molecular Weight
- 405.96 g/mol
- Moisture (%)
- Not more than 1.0%
- EINECS No
- 213-228-6
- Assay
- Not less than 98.0% and not more than 102.0% (on dry basis)
- Loss on Drying
- Not more than 1.0%
- Place of Origin
- India
- Melting Point
- 116-118C
- Storage
- Store in a cool, dry and well-ventilated place in tightly closed containers
- Heavy Metal (%)
- Not more than 0.001%
- Ph Level
- Neutral
- Molecular Formula
- C26H28ClNO
- Other Names
- Clomifene; Clomid; Serophene
- CAS No
- 911-45-5
- Type
- Active Pharmaceutical Ingredient
- Grade
- Pharmaceutical Grade
- Usage
- Used in female infertility treatment
- Purity
- 99% min
- Appearance
- White to off-white crystalline powder
- Application
- Fertility drug or ovulation inducer
- Raw Material
- Yes
- Smell
- Odorless
- Color
- White to off-white
- Form
- Powder
- Packaging
- Double polyethylene bags placed in fiber drums of 25 kg net each
- Impurity Level
- Total impurities NMT 0.5%
- Stability
- Stable under recommended storage conditions
- Shelf Life
- 36 months
- Identification
- By IR and UV spectroscopy, conforms to standard
- Specific Rotation
- Between -115° to -125° (in specified solvent)
- Solubility
- Slightly soluble in water, freely soluble in methanol and ethanol
- Country of Export
- India
- Manufacturing Standard
- USP
Clomiphene Citrate USP Trade Information
- Minimum Order Quantity
- 5 Kilograms
- Payment Terms
- Cash Against Delivery (CAD), Cash on Delivery (COD), Letter of Credit (L/C), Cash in Advance (CID), Cheque, Cash Advance (CA)
- Supply Ability
- 10000 Kilograms Per Month
- Delivery Time
- 15 Days
- Sample Available
- Yes
- Main Domestic Market
- All India
- Certifications
- GMP
About Clomiphene Citrate USP
Banking upon in-depth domain knowledge and years of vast experience, we are engaged in offering Clomiphene Chemicals. These chemicals are triphenyl ethylene stilbene derivative. To process these chemicals, our skilled workforce uses best quality compounds, sourced from trusted vendors. These are used mainly in female infertility due to anovulation to induce ovulation. Clomiphene Chemicals are orally administered, and they act as a selective estrogen receptor modulator (SERM). They have both estrogenic and anti-estrogenic properties. These chemicals stumulate the release of gonadotropins, follicle-stimulating hormone and leuteinizing hormone. Packed in quality grade containers, these chemicals are available in different quantity packaging options.
Stringent Quality & Safety Standards
Clomiphene Citrate USP adheres to rigorous manufacturing and quality benchmarks, conforming to identification by IR and UV spectroscopy and impurity levels not exceeding 0.5%. Heavy metals, moisture, and other contaminants are strictly controlled, ensuring pharmaceutical-grade quality and stability throughout its 36-month shelf life.
Packaging and Storage Solutions
To maintain its integrity, each batch of Clomiphene Citrate USP is packaged in double polyethylene bags placed inside fiber drums, each holding 25 kg net. For optimal stability, products should be stored in cool, dry, well-ventilated spaces with containers tightly closed, effectively safeguarding the API against moisture and environmental exposure.
Applications and Intended Use
As a pivotal fertility drug, Clomiphene Citrate is widely utilized to induce ovulation in women experiencing infertility. Its proven efficacy and safety profile make it a preferred raw material for pharmaceutical formulations designed for female reproductive therapies. The product is suitable for various dosage forms after compounding by qualified professionals.
FAQs of Clomiphene Citrate USP:
Q: How should Clomiphene Citrate USP be stored to maintain its stability?
A: Clomiphene Citrate USP should be stored in a cool, dry, and well-ventilated area, with tightly closed containers to prevent moisture ingress. This ensures maximum stability and a shelf life of up to 36 months.Q: What is the recommended method for identifying Clomiphene Citrate USP?
A: Identification of Clomiphene Citrate USP is performed using IR (Infrared) and UV (Ultraviolet) spectroscopy, in alignment with USP regulatory standards, to confirm its authenticity and purity.Q: When is Clomiphene Citrate USP typically used in pharmaceutical applications?
A: This API is primarily used in the formulation of fertility medications for women, particularly to induce ovulation as part of infertility treatments. It must be compounded into dosage forms by pharmaceutical professionals.Q: Where is Clomiphene Citrate USP manufactured and exported from?
A: Clomiphene Citrate USP is manufactured and exported from India, following strict pharmaceutical guidelines to ensure global regulatory compliance.Q: What are the benefits of using Clomiphene Citrate USP for fertility treatments?
A: Clomiphene Citrate USP assists in stimulating ovulation in women with infertility issues, increasing the likelihood of conception. Its high purity and stringent manufacturing standards offer consistent and reliable therapeutic outcomes.Q: What is the solubility profile of Clomiphene Citrate USP, and how does it impact formulation?
A: Clomiphene Citrate USP is slightly soluble in water but freely soluble in methanol and ethanol. This solubility profile enables its effective incorporation into various pharmaceutical formulations by skilled professionals.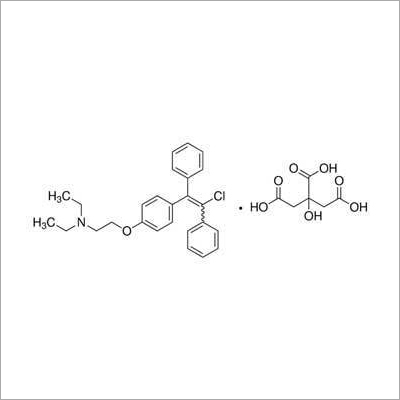

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Clomiphene Citrate Category
Clomiphene Citrate BP
Minimum Order Quantity : 5 Kilograms
Application : Other, Fertility drug / Ovulation inducer
Color : White
Other Names : Clomifene citrate, Clomifene, Clomid, Serophene, Omifin
Residue on Ignition : Not more than 0.2%
Purity : Not less than 98.0%
Clomiphene Citrate IP
Minimum Order Quantity : 5 Kilograms
Application : Other, Fertility treatment, API for pharmaceuticals
Color : White
Other Names : Clomifene Citrate, Clomid
Residue on Ignition : Not more than 0.1%
Purity : 99% min
GST : 24AABCP1568Q1Z0
|
 |
PALAM PHARMA PVT. LTD.
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |


 Send Inquiry
Send Inquiry