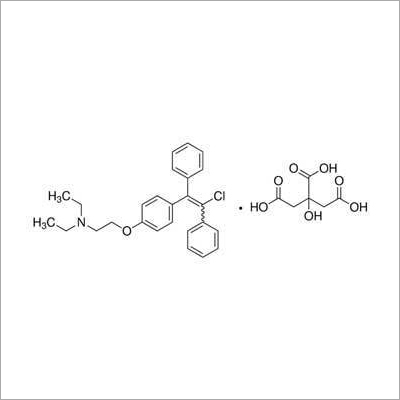
Clomiphene Citrate IP
Clomiphene Citrate IP Specification
- Storage
- Store below 30C, protected from light and moisture
- Molecular Weight
- 598.09 g/mol
- Moisture (%)
- Not more than 0.5%
- Heavy Metal (%)
- Not more than 0.001%
- Ph Level
- Not less than 4.5 and not more than 5.5 (1% aqueous solution)
- Loss on Drying
- Not more than 0.5%
- Molecular Formula
- C26H28ClNOC6H8O7
- HS Code
- 29379000
- Place of Origin
- India
- Particle Size
- 95% passes through 60 mesh
- Residue on Ignition
- Not more than 0.1%
- Assay
- 99%
- Other Names
- Clomifene Citrate, Clomid
- Type
- Pharmaceutical Raw Material
- Grade
- IP (Indian Pharmacopoeia)
- Purity
- 99% min
- Appearance
- White or almost white crystalline powder
- Application
- Fertility treatment, API for pharmaceuticals
- Raw Material
- Yes
- Smell
- Odorless
- Color
- White
- Form
- Powder
Clomiphene Citrate IP Trade Information
- Minimum Order Quantity
- 5 Kilograms
- Payment Terms
- Cash on Delivery (COD), Cash Against Delivery (CAD), Cash in Advance (CID), Cheque, Cash Advance (CA)
- Supply Ability
- 10000 Kilograms Per Month
- Delivery Time
- 15 Days
- Main Domestic Market
- All India
About Clomiphene Citrate IP
Holding a highly reputed and trusted market standing, we are engaged in offering our clients Clomiphene Citrate USP Chemicals. These medications are used to treat infertility in women. They work by stimulating an increase in the amount of hormones that support the growth and release of a mature egg. We formulate these chemicals under the strict inspection of expert supervisors using high quality compounds. These medications are orally administered, non steroidal, ovulatory stimulant that act as a selective estrogen receptor modulator (SERM). Clomiphene Citrate USP Chemicals are known for having both estrogenic and anti-estrogenic properties.
Name of Product: CLOMIPHENE CITRATE BP
Formula: C26H28ClNO,C6H8O7
Mol. Wt.: 598.
| Analysis | Specification |
| Description | White or Pale yellow, crystalline powder |
| Solubility | Slightly soluble in water, sparingly soluble in alcohol |
| Identification: A: B: | By infra-red absorption spectrum A deep red Colour is produced. |
| Water | Not More than 1.0 % |
| Related Substances | Not more than 2.5 % ( Total Impurity) |
| Z-ISOMER | Between 30.00 % and 50.00 % |
| Assay | Clomiphene citrate contains not less than 98.0 per cent and not more than 101.0 per cent of calculated on the anhydrous substance. |
Category: Used Mainly in Female Infertility Due to Anovulation.
Premium Quality and Purity
Clomiphene Citrate IP stands out with a minimum purity of 99%, ensuring consistent and reliable performance in pharmaceutical applications. Its crystalline powder form is odorless, with 95% of particles passing through a 60 mesh, making it ideal for precise formulations. Meeting Indian Pharmacopoeia standards, it guarantees low moisture and minimal impurity levels.
Primary Applications and Uses
This high-purity raw material is an essential API in numerous fertility medications. Clomiphene Citrate is widely used in the treatment of infertility, aiding hormonal stimulation in patients. Being a pharmaceutical-grade substance, it can be seamlessly integrated into medicinal products by manufacturers. Exported and supplied from India, it upholds strict quality control measures.
Safe Handling and Storage
Store Clomiphene Citrate below 30C in a cool, dry place, away from light and moisture, to maintain its stability and effectiveness. Proper handling ensures the powder retains its quality, preventing degradation and potential contamination. With heavy metals and residue kept to a minimum, safety and purity are prioritized for end users and manufacturers alike.
FAQs of Clomiphene Citrate IP:
Q: How is Clomiphene Citrate IP typically used in pharmaceutical manufacturing?
A: Clomiphene Citrate IP serves as an active pharmaceutical ingredient (API) in the formulation of fertility medications, particularly for stimulating ovulation in individuals facing infertility. It is blended into tablet or capsule forms under strictly controlled conditions by pharmaceutical manufacturers.Q: What distinguishes Clomiphene Citrate IP in terms of purity and quality?
A: With a minimum purity level of 99% and compliance with Indian Pharmacopoeia standards, Clomiphene Citrate IP is a premium-grade material. It is nearly odorless and white in appearance, ensuring minimal impurities and tight control of moisture and residue levels for pharmaceutical safety.Q: When should Clomiphene Citrate powder be stored under special conditions?
A: Clomiphene Citrate should always be stored below 30C, protected from light and moisture. These conditions preserve its potency, structural integrity, and prevent any degradation or contamination of the raw material.Q: Where is Clomiphene Citrate IP manufactured and exported from?
A: This high-quality pharmaceutical raw material is manufactured and exported from India, adhering to the rigorous standards of the Indian Pharmacopoeia (IP) for international supply.Q: What is the process for ensuring Clomiphene Citrates particle size suitability?
A: Through controlled milling and sieving processes, 95% of Clomiphene Citrate IP powder passes through a 60 mesh sieve. This guarantees a consistent particle size suitable for pharmaceutical applications.Q: What benefits does Clomiphene Citrate IP offer to pharmaceutical manufacturers?
A: Pharmaceutical companies benefit from its superior purity, tight specification compliance, and ease of formulation. Its reliability as an API supports the development of effective fertility treatments and facilitates regulatory approvals.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Clomiphene Citrate Category
Clomiphene Citrate USP
Minimum Order Quantity : 5 Kilograms
Application : Other, Fertility drug or ovulation inducer
Residue on Ignition : Not more than 0.1%
Raw Material : Yes
Appearance : White to offwhite crystalline powder
HS Code : 29333990
Clomiphene Citrate BP
Minimum Order Quantity : 5 Kilograms
Application : Other, Fertility drug / Ovulation inducer
Residue on Ignition : Not more than 0.2%
Raw Material : Yes
Appearance : White or almost white crystalline powder
HS Code : 29339900
GST : 24AABCP1568Q1Z0
|
 |
PALAM PHARMA PVT. LTD.
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |


 Send Inquiry
Send Inquiry